Lipid (B)
Introduction
|
Introduction
Introduction (B)
Lipid, any of a diverse group of organic compounds including fats, oils, hormones, and certain components of membranes that are grouped together because they do not interact appreciably with water. One type of lipid, the triglycerides, is sequestered as fat in adipose cells, which serve as the energy-storage depot for organisms and also provide thermal insulation. Some lipids such as steroid hormones serve as chemical messengers between cells, tissues, and organs, and others communicate signals between biochemical systems within a single cell. The membranes of cells and organelles (structures within cells) are microscopically thin structures formed from two layers of phospholipid molecules. Membranes function to separate individual cells from their environments and to compartmentalize the cell interior into structures that carry out special functions. So important is this compartmentalizing function that membranes, and the lipids that form them, must have been essential to the origin of life itself. |
| |
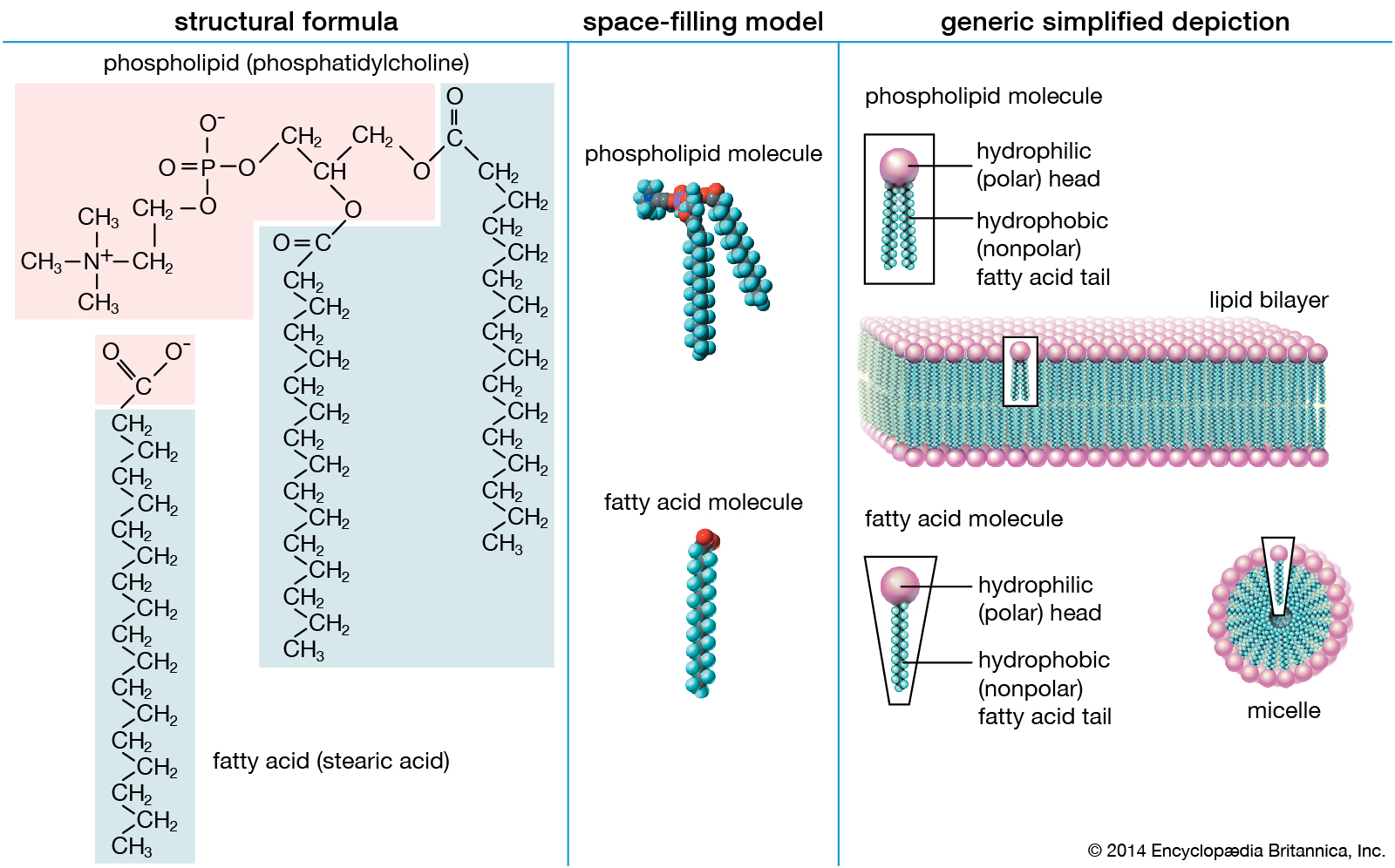
Structure and properties of two representative lipids. Both stearic acid (a fatty acid) and phosphatidylcholine (a phospholipid) are composed of chemical groups that form polar “heads” and nonpolar “tails.” The polar heads are hydrophilic, or soluble in water, whereas the nonpolar tails are hydrophobic, or insoluble in water. Lipid molecules of this composition spontaneously form aggregate structures such as micelles and lipid bilayers, with their hydrophilic ends oriented toward the watery medium and their hydrophobic ends shielded from the water. |
|
| |
| Water is the biological milieu—the substance that makes life possible—and almost all the molecular components of living cells, whether they be found in animals, plants, or microorganisms, are soluble in water. Molecules such as proteins, nucleic acids, and carbohydrates have an affinity for water and are called hydrophilic (“water-loving”). Lipids, however, are hydrophobic (“water-fearing”). Some lipids are amphipathic—part of their structure is hydrophilic and another part, usually a larger section, is hydrophobic. Amphipathic lipids exhibit a unique behaviour in water: they spontaneously form ordered molecular aggregates, with their hydrophilic ends on the outside, in contact with the water, and their hydrophobic parts on the inside, shielded from the water. This property is key to their role as the fundamental components of cellular and organelle membranes. |
| |
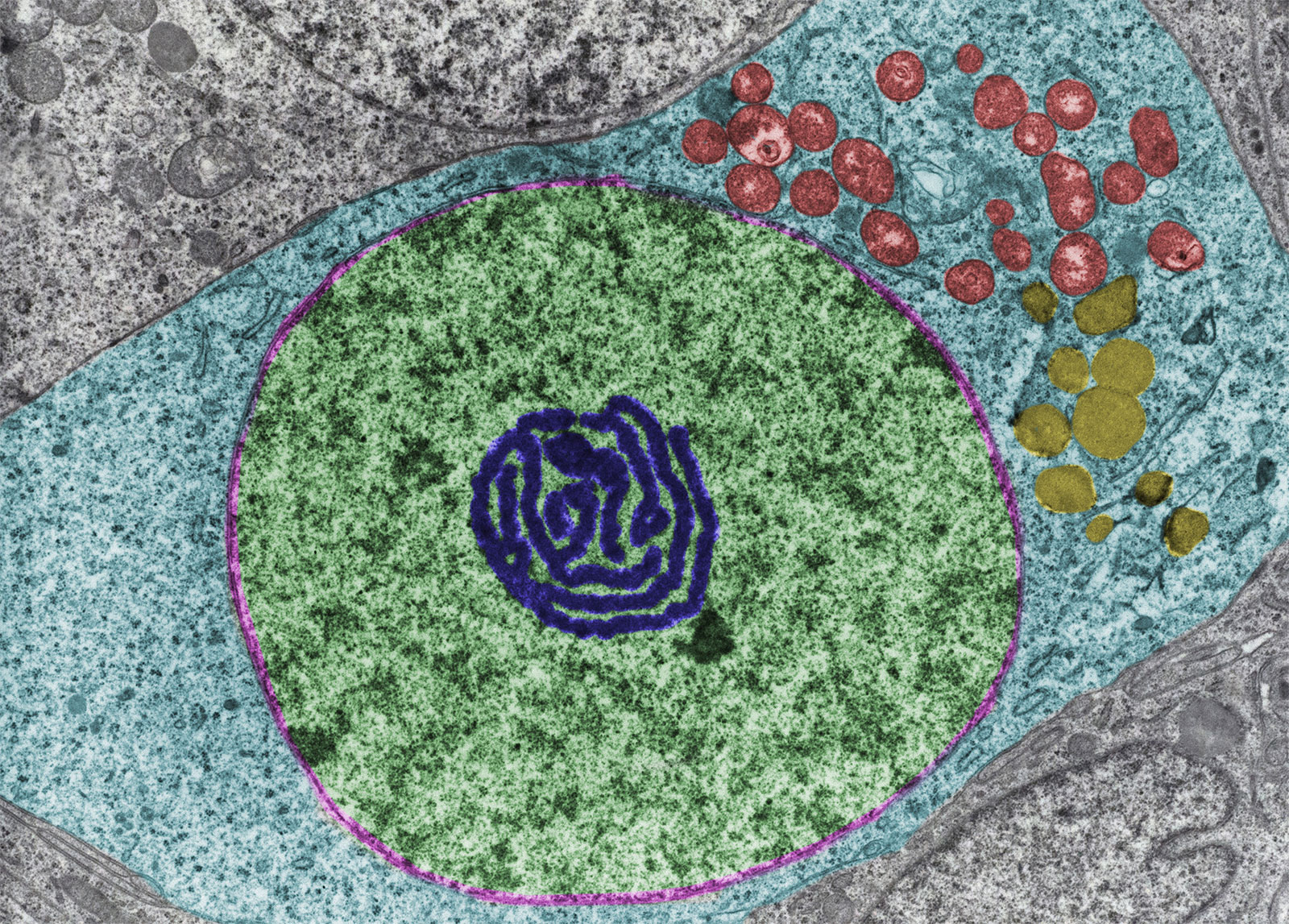
Lipid; oogonium
A false colour transmission electron microscope micrograph of an oogonium (an egg cell of certain algae and fungi), showing an abundance of lipid droplets (yellow), a nucleus (green), an atypical nucleolus (dark blue), and mitochondria (red). |
|
| |
| Although biological lipids are not large macromolecular polymers (e.g., proteins, nucleic acids, and polysaccharides), many are formed by the chemical linking of several small constituent molecules. Many of these molecular building blocks are similar, or homologous, in structure. The homologies allow lipids to be classified into a few major groups: fatty acids, fatty acid derivatives, cholesterol and its derivatives, and lipoproteins. This article covers the major groups and explains how these molecules function as energy-storage molecules, chemical messengers, and structural components of cells. |

|
|
|
|
| |
Fatty Acids
|
Fatty Acids
Fatty Acids (B)
Fatty acids rarely occur as free molecules in nature but are usually found as components of many complex lipid molecules such as fats (energy-storage compounds) and phospholipids (the primary lipid components of cellular membranes). This section describes the structure and physical and chemical properties of fatty acids. It also explains how living organisms obtain fatty acids, both from their diets and through metabolic breakdown of stored fats. |
|
|
|
Structure
Structure (B)
|
|
| |
|
| Structural formula of stearic acid. |
|
| |
|
Biological fatty acids, members of the class of compounds known as carboxylic acids, are composed of a hydrocarbon chain with one terminal carboxyl group (COOH). The fragment of a carboxylic acid not including the hydroxyl (OH) group is called an acyl group. Under physiological conditions in water, this acidic group usually has lost a hydrogen ion (H+) to form a negatively charged carboxylate group (COO−). Most biological fatty acids contain an even number of carbon atoms because the biosynthetic pathway common to all organisms involves chemically linking two-carbon units together (although relatively small amounts of odd-number fatty acids do occur in some organisms). Although the molecule as a whole is water-insoluble by virtue of its hydrophobic hydrocarbon chain, the negatively charged carboxylate is hydrophilic. This common form for biological lipids—one that contains well-separated hydrophobic and hydrophilic parts—is called amphipathic.
In addition to straight-chain hydrocarbons, fatty acids may also contain pairs of carbons linked by one or more double bonds, methyl branches, or a three-carbon cyclopropane ring near the centre of the carbon chain. |

|
|
|
|
Saturated fatty acids
Saturated fatty acids (B)
The simplest fatty acids are unbranched, linear chains of CH2 groups linked by carbon-carbon single bonds with one terminal carboxylic acid group. The term saturated indicates that the maximum possible number of hydrogen atoms are bonded to each carbon in the molecule. Many saturated fatty acids have a trivial or common name as well as a chemically descriptive systematic name. The systematic names are based on numbering the carbon atoms, beginning with the acidic carbon. The table gives the names and typical biological sources of the most common saturated fatty acids. Although the chains are usually between 12 and 24 carbons long, several shorter-chain fatty acids are biochemically important. For instance, butyric acid (C4) and caproic acid (C6) are lipids found in milk. Palm kernel oil, an important dietary source of fat in certain areas of the world, is rich in fatty acids that contain 8 and 10 carbons (C8 and C10). |
| |
Common saturated fatty acids
| trivial name |
systematic name |
number of carbons in chain |
typical sources |
| lauric acid |
n-dodecanoic acid |
12 |
palm kernel oil, nutmeg |
| myristic acid |
n-tetradecanoic acid |
14 |
palm kernel oil, nutmeg |
| palmitic acid |
n-hexadecanoic acid |
16 |
olive oil, animal lipids |
| stearic acid |
n-octadecanoic acid |
18 |
cocoa butter, animal lipids |
| behenic acid |
n-docosanoic acid |
22 |
brain tissue, radish oil |
| lignoceric acid |
n-tetracosanoic acid |
24 |
brain tissue, carnauba wax |
|

|
|
|
|
Unsaturated fatty acids
Unsaturated fatty acids (B)
Unsaturated fatty acids have one or more carbon-carbon double bonds. The term unsaturated indicates that fewer than the maximum possible number of hydrogen atoms are bonded to each carbon in the molecule. The number of double bonds is indicated by the generic name—monounsaturated for molecules with one double bond or polyunsaturated for molecules with two or more double bonds. Oleic acid is an example of a monounsaturated fatty acid. Common representative monounsaturated fatty acids together with their names and typical sources are listed in the table. The prefix cis-9 in the systematic name of palmitoleic acid denotes that the position of the double bond is between carbons 9 and 10. Two possible conformations, cis and trans, can be taken by the two CH2 groups immediately adjacent to the double-bonded carbons. In the cis configuration, the one occurring in all biological unsaturated fatty acids, the two adjacent carbons lie on the same side of the double-bonded carbons. In the trans configuration, the two adjacent carbons lie on opposite sides of the double-bonded carbons. |
| |
Common monounsaturated fatty acids
| trivial name |
systematic name |
number of carbons in chain |
typical sources |
| palmitoleic acid |
cis-9-hexadecenoic acid |
16 |
marine algae, pine oil |
| oleic acid |
cis-9-octadecenoic acid |
18 |
animal tissues, olive oil |
| gadoleic acid |
cis-9-eicosenoic acid |
20 |
fish oils (cod, sardine) |
| erucic acid |
cis-13-docosenoic acid |
22 |
rapeseed oil |
| nervonic acid |
cis-15-tetracosenoic acid |
24 |
sharks, brain tissue |
|
| |
|
|
| |
|
| Structural formula of oleic acid. |
|
| |
|
Fatty acids containing more than one carbon-carbon double bond ( polyunsaturated fatty acids) are found in relatively minor amounts. The multiple double bonds are almost always separated by a CH2 group (―CH2―CH=CH―CH2―CH=CH―CH2―), a regular spacing motif that is the result of the biosynthetic mechanism by which the double bonds are introduced into the hydrocarbon chain. The table lists the most common polyunsaturated fatty acids, linoleic and arachidonic, together with several that are less common. Arachidonic acid (C20) is of particular interest as the precursor of a family of molecules, known as eicosanoids (from Greek eikosi, “twenty”), that includes prostaglandins, thromboxanes, and leukotrienes. These compounds, produced by cells under certain conditions, have potent physiological properties, as explained in the section Intracellular and extracellular messengers. Animals cannot synthesize two important fatty acids, linoleic acid (an omega-6 fatty acid) and alpha-linolenic acid (an omega-3 fatty acid), that are the precursors of the eicosanoids and so must obtain them in the diet from plant sources. For this reason, these precursors are called essential fatty acids. |
| |
Common polyunsaturated fatty acids
| trivial name |
systematic name |
number of carbons in chain |
typical sources |
| linoleic acid |
cis-9-, cis-12-octadecadienoic acid |
18 |
corn oil, animal tissues, bacteria |
| linolenic acid |
cis-9-, cis-12-, cis-15-octadecatrienoic acid |
18 |
animal tissues |
| 5,8,11-eicosatrienoic acid |
20 |
|
| 8,11,14-eicosatrienoic acid |
20 |
brain tissue |
| 7,10,13-docosatrienoic acid |
22 |
phospholipids |
| 8,11,14-docosatrienoic acid |
22 |
|
| arachidonic acid |
5,8,11,14-eicosatetraenoic acid |
20 |
liver, brain tissue |
| 4,7,10,13-docosatetraenoic acid |
22 |
brain tissue |
| 4,7,10,13,16,19-docosahexaenoic acid |
22 |
brain tissue |
|
| |
| Trans polyunsaturated fatty acids, although not produced biosynthetically by mammals, are produced by microorganisms in the gut of ruminant animals such as cows and goats, and they are also produced synthetically by partial hydrogenation of fats and oils in the manufacture of margarine (the so-called trans fats). There is evidence that ingestion of trans fats can have deleterious metabolic effects. |

|
|
|
|
Substituent groups
Substituent groups (B)
In addition to the very common fatty acids with straight saturated or unsaturated acyl chains, many fatty acids are chemically modified by substituents on the hydrocarbon chain. For example, the preening gland of ducks secretes a fatty acid 10 carbons long with methyl (CH3) groups substituted for one of the hydrogens on carbons 2, 4, 6, and 8. Some bacteria produce fatty acids that have a methyl group on the carbon atom farthest from the acidic group or on the penultimate carbon. Other bacteria incorporate a cyclopropane ring near the centre of the acyl chain. The bacterium that causes tuberculosis (Mycobacterium tuberculosis) synthesizes a whole family of cyclopropane-containing fatty acids called α-mycolic acids. Similar fatty acids are found in related bacteria. A third common constituent is a hydroxyl group (OH). Monohydroxyl acids are found in both plants and animals in relatively small amounts, but they are more prevalent in bacteria. |
|
|
|
Physical properties
Physical properties (B)
Pure fatty acids form crystals that consist of stacked layers of molecules, with each layer the thickness of two extended molecules. The molecules in a layer are arranged so that the hydrophobic (water-fearing) hydrocarbon chains form the interior of the layer and the hydrophilic (water-loving) carboxylic acid groups form the two faces. For a specific fatty acid the details of the molecular packing may vary, giving rise to different crystal forms known as polymorphs.
The melting temperatures of saturated fatty acids of biological interest are above 27 °C (81 °F) and rise with increasing length of the hydrocarbon chain. Monounsaturated and polyunsaturated molecules melt at substantially lower temperatures than do their saturated analogs, with the lowest melting temperatures occurring when the carbon-carbon double bonds are located near the centre of the hydrocarbon chain, as they are in most biological molecules. As a result, these molecules form viscous liquids at room temperature.
The hydrophobic character of the hydrocarbon chain of most biological fatty acids exceeds the hydrophilic nature of the carboxylic acid group, making the water solubility of these molecules very low. For example, at 25 °C (77 °F) the solubility in grams of fatty acid per gram of solution is 3 × 10−6. Water solubility decreases exponentially with the addition of each carbon atom to the hydrocarbon chain. This relationship reflects the energy required to transfer the molecule from a pure hydrocarbon solvent to water. With each CH2 group, for instance, more energy is required to order water molecules around the hydrocarbon chain of the fatty acid, which results in the hydrophobic effect.
In pure water the carboxylate group can dissociate a positively charged hydrogen ion to only a very small degree thus:
R―COOH → RCOO− + H+.
Here R represents the hydrocarbon chain. The carboxylate ion, bearing a negative charge, is more polar than the undissociated acid. RCOOH can be converted completely to the ion RCOO− by adding an equal number of molecules of a base such as sodium hydroxide (NaOH). This effectively replaces the H+ with Na+ to give the salt of the fatty acid, which is a soap. The very useful detergent property of soaps stems from the fact that the RCOO− anions in water spontaneously form stable, spherical aggregates called micelles. The interior of these structures, formed by the hydrocarbon chains, is an excellent solvent in which grease and hydrophobic dirt of all sorts can be sequestered. The diameter of each micelle is roughly twice the length of the extended fatty acid. Dispersions of micelles in water can be made quite concentrated and exhibit great cleansing power. These dispersions are stable and generally look very much like pure water. Bubbles and foams on the surface of soap dispersions are the result of the spontaneous adsorption of RCOO− ions at the interface between the aqueous dispersion and air, with the result that the air-water interfaces are energetically stabilized and can therefore be mechanically expanded. |
| |
|
|
| |
|
| When a soap is dissolved in water, fatty acids in the soap form spherical structures called micelles, in which the hydrophilic “heads” of the fatty acid molecules are turned toward the water and the hydrophobic “tails” are sheltered in the interior. |
|
|

|
|
|
|
Chemical properties
Chemical properties (B)
The most chemically reactive portion of fatty acids is the acidic carboxyl group (COOH). It reacts with alcohols (R′OH) to form products known as esters (RCOOR′) and releases water in the process. This ester bond is the principal covalent bond linking fatty acid moieties to other groups in the more-complex lipids discussed in other sections of this article. A second chemical bond, occurring much less frequently in biological lipids involving fatty acids, is the ether bond (R′―O―R). Ether bonds are chemically more stable than ester bonds.
The hydrocarbon part of a fatty acid molecule is quite resistant to chemical attack unless carbon-carbon double bonds are present. A number of different kinds of molecules react with such a double bond. For example, when a catalyst such as platinum is present, hydrogen gas adds to the double bond to give a saturated fatty acid. Halogens (chlorine, bromine, and iodine) and their derivatives such as hydroiodic acid (HI) also react with the double bond to form saturated fatty acids, but in these cases one or two atoms of the halogen replace one or two of the hydrogens normally found in the saturated acyl chain. Carbon-carbon double bonds can also react with oxygen in either nonenzymatic processes or enzymatically catalyzed oxidation reactions. This process generates a variety of products, some of which contribute to the rancid smell in spoiled meat and vegetable products. In general, the more highly unsaturated the fatty acid, the more easily it is oxidized. |

|
|
|
|
Biological sources
Biological sources (B)
Fatty acids are found in biological systems either as free molecules or as components of more-complex lipids. They are derived from dietary sources or produced by metabolism, as described below. |
|
|
|
Digestion of dietary fatty acids
Digestion of dietary fatty acids (B)
The main source of fatty acids in the diet is triglycerides, generically called fats. In humans, fat constitutes an important part of the diet, and in some countries it can contribute as much as 45 percent of energy intake. Triglycerides consist of three fatty acid molecules, each linked by an ester bond to one of the three OH groups of a glycerol molecule. After ingested triglycerides pass through the stomach and into the small intestine, detergents called bile salts are secreted by the liver via the gall bladder and disperse the fat as micelles. Pancreatic enzymes called lipases then hydrolyze the dispersed fats to give monoglycerides and free fatty acids. These products are absorbed into the cells lining the small intestine, where they are resynthesized into triglycerides. The triglycerides, together with other types of lipids, are then secreted by these cells in lipoproteins, large molecular complexes that are transported in the lymph and blood to recipient organs. In detail, the process of triglyceride or fat absorption from dietary sources is quite complex and differs somewhat depending upon the animal species. |

|
|
|
|
Storage
Storage (B)
After transport through the circulation, triglycerides are hydrolyzed yet again to fatty acids in the adipose tissue. There they are transported into adipose cells, where once again they are resynthesized into triglycerides and stored as droplets. Fat or adipose tissue essentially consists of cells, whereby the interior of each cell is largely occupied by a fat droplet. The triglyceride in these droplets is available to the body on demand as communicated to adipose tissue by hormone messengers.
Various animals store triglycerides in different ways. In sharks, for example, fat is stored in the liver, but in bony fish it is deposited in and around muscle fibres. Insects store fat in a special organ called the fat body. The development of true adipose tissue is found only in higher animals.
|
|
|
|
Biosynthesis
Biosynthesis (B)
In mammals, fatty acids are synthesized in adipose and liver cells from glucose via a fairly complex pathway. In essence, the six carbons of a glucose molecule are oxidized to a pair of two-carbon carboxylic acid fragments called acetate. The starting point for biosynthesis is an acetate group chemically linked to a molecule of CoA (coenzyme A). The process of building up the acyl chain of a fatty acid then begins, basically through the sequential chemical addition of two-carbon fragments from CoA-acetate to generate, for example, the 16-carbon saturated fatty acid palmitate. This process is catalyzed by a complex enzyme known as fatty acid synthase. Elongation of the palmitate carbon chain and the introduction of carbon-carbon double bonds are carried out subsequently by other enzyme systems. The overall process is basically the same in organisms ranging from bacteria to humans. |
|
|
|
| |
Fatty Acid Derivatives
|
Triglycerides
Triglycerides (B)
No text. |
|
|
|
Structure
Structure (B)
Triglycerides (chemical name triacylglycerol), the principal means of storing fatty acids in biological systems, are a class of compounds that consist of glycerol (a three-carbon trihydroxy alcohol) with a fatty acid linked to each of the three OH groups by an ester bond. An example of a typical triglyceride is tristearin. Because this molecule contains only one type of fatty acid, it is referred to as a simple triglyceride. Almost all naturally occurring triglyceride molecules, however, contain more than one type of fatty acid; when two or more in a given molecule are different, it is called a mixed triglyceride. For any specific combination of three fatty acids, three different molecules are possible, depending upon which of the three fatty acids is bonded to the central carbon of glycerol. Considering the numbers of common saturated, monounsaturated, and polyunsaturated fatty acids, it is evident that there are a great many different triglycerides. |
| |
|
|
| |
|
| Structural formula of tristearin (tristearic acid). |
|
|

|
|
|
|
Physical properties
Physical properties (B)
Triglycerides are hydrophobic substances that are soluble only in some organic solvents. Unlike many other types of complex lipids, they possess no electric charges and are therefore referred to as neutral lipids. The molecular structure of a few triglycerides that have been studied in crystals indicates that the acyl chains on the 1 and 2 carbons of glycerol, together with the 1 and 2 carbons of glycerol itself, form a straight line. Carbon 3 projects at right angles to this line, but the acyl chain on its glycerol folds over at the carboxyl carbon to lie alongside the acyl chain on carbon 1. Triglyceride molecules look much like a tuning fork and, when packed together, produce layered crystals.
The melting temperatures of mixed triglycerides are roughly an average of the melting temperatures of their constituent fatty acids. In simple triglycerides, melting temperatures rise with increasing acyl chain length but drop with increasing number of double bonds. Melted triglycerides are generally quite viscous oils. From the physiological standpoint, it is important that most stored triglycerides be fluid at body temperature in order to permit their rapid mobilization as an energy source. Liquidity is also important since subcutaneous stored fats perform an insulating function that must not interfere with the mobility of the organism and its parts. |

|
|
|
|
Waxes
Waxes (B)
A second group of neutral lipids that are of physiological importance, though they are a minor component of biological systems, are waxes. Essentially, waxes consist of a long-chain fatty acid linked through an ester oxygen to a long-chain alcohol. These molecules are completely water-insoluble and generally solid at biological temperatures. Their strongly hydrophobic nature allows them to function as water repellents on the leaves of some plants, on feathers, and on the cuticles of certain insects. Waxes also serve as energy-storage substances in plankton (microscopic aquatic plants and animals) and in higher members of the aquatic food chain. Plankton apparently use the biosynthesis of waxes to adjust their buoyant density and thus their depth in the ocean. It has been suggested that a major source of petroleum found in deep-sea sediments originates from the deposition of wax-rich dead plankton over vast periods of time. Whales and many fishes also store large quantities of waxes. |

|
|
|
|
Biological membrane lipids
Biological membrane lipids (B)
The three principal classes of lipids that form the bilayer matrix of biological membranes are glycerophospholipids, sphingolipids, and sterols (principally cholesterol). The most important characteristic of molecules in the first two groups is their amphipathic structure—well separated hydrophilic (polar) and hydrophobic (nonpolar) regions. Generally, their shape is elongated, with a hydrophilic end or head attached to a hydrophobic moiety by a short intervening region of intermediate polarity. Because of the segregation of polarity and nonpolarity, amphipathic molecules in any solvent will spontaneously form aggregates that minimize energetically unfavourable contacts (by keeping unlike regions of molecules apart) and maximize favourable contacts with the solvent (by keeping similar regions of molecules together). The molecular arrangement of the aggregate depends upon the solvent and the details of the amphipathic structure of the lipid.
In water, micelles formed by soaps (the sodium or potassium salts of fatty acids) are one such aggregate. The polar or hydrophilic portion of the soap molecules forms the surface of the micelle, while the hydrocarbon chains form its interior and are thus completely protected from the energetically unfavourable contact with water, as described in the section Fatty acids: Physical properties. Biological membrane lipids, however, do not form spherical micelles in water but instead form topologically closed lamellar (layered) structures. The polar heads of the component molecules form the two faces of the lamella, while the hydrophobic moieties form its interior. Each lamella is thus two molecules in thickness, with the long axis of the component molecules perpendicular to the plane of the bilayer. |
| |
|
|
| |
|
| Lipid bilayer; cell membranePhospholipid molecules, like molecules of many lipids, are composed of a hydrophilic “head” and one or more hydrophobic “tails.” In a water medium, the molecules form a lipid bilayer, or two-layered sheet, in which the heads are turned toward the watery medium and the tails are sheltered inside, away from the water. This bilayer is the basis of the membranes of living cells. |
|
|
| |
| Other types of aggregates are also formed in water by certain amphipathic lipids. For example, liposomes are artificial collections of lipids arranged in a bilayer, having an inside and an outside surface. The lipid bilayers form a sphere that can trap a molecule inside. The liposome structure can be useful for protecting sensitive molecules that are to be delivered orally. |
| |
|
|
| |
|
| Phospholipids can be used to form artificial structures called liposomes, which are double-walled hollow spheres useful for encapsulating other molecules such as pharmaceutical drugs. |
|
|

|
|
|
|
Glycerophospholipids
Glycerophospholipids (B)
Lipids of this class are the most abundant in biological membranes. In glycerophospholipids, fatty acids are linked through an ester oxygen to carbons 1 and 2 of glycerol, the backbone of the molecule. Phosphate is ester-linked to carbon 3, while any one of several possible substituents is also linked to the phosphate moiety. Glycerophospholipids are amphipathic—glycerol and phosphate form the polar end of the molecule, while hydrocarbon chains form the nonpolar end. Although the fatty acids can be any of those common in biological systems, usually those attached to carbon 1 are saturated and those attached to carbon 2 are unsaturated. The various combinations of two fatty acids give rise to many different molecules bearing the same substituent group. Since this is true for each head group, there are altogether about a thousand possible types of glycerophospholipids. The great majority are found in biological membranes. |
| |
|
|
| |
|
Glycerophospholipid structure
General structural formula of a glycerophospholipid. The composition of the specific molecule depends on the chemical group (designated R3 in the diagram) linked to the phosphate and glycerol “head” and also on the lengths of the fatty acid “tails” (R1 and R2). |
|
|
| |
From the standpoint of physical properties, the greatest difference among various molecules lies in the particular substituent. This is due in part to the different sizes of the various types and in part to differences in their electric charges. The phosphatidylcholines and phosphatidylethanolamines are zwitterionic, meaning they have one negative and one positive charge on the substituent group. Phosphatidic acid, phosphatidylserine, and phosphatidylinositol have a net negative charge. Differences in fatty acid composition also contribute to differences in physical properties of a series of molecules with the same substituent. In the presence of an excess of water, the molecules form aggregates with a variety of geometries, the most common of which is the bilayer.
In bilayers many glycerophospholipids as well as sphingomyelin (discussed below) can be in either one of two states, gel or liquid-crystalline. In the solidlike gel state, the lipid molecules in each half of the bilayer are arranged in a two-dimensional lattice, with their two acyl chains in the extended form. With the application of heat, the gel converts into the liquid-crystalline state at some temperature characteristic of the lipid mixture. In this state the molecules in each half of the bilayer remain in a fairly regular two-dimensional lattice but are free to rotate about their long axes and slide laterally through the layer. Their acyl chains now undergo considerable motion, leading to transiently kinked conformations. These motions give the bilayer a quasi-liquid behaviour that is characteristic of the bilayers in all biological membranes. |

|
|
|
|
Sphingolipids
Sphingolipids (B)
A second major class of lipids usually associated with the membranes surrounding cells is sphingolipids. Sphingolipids are based on an 18-carbon amine alcohol, sphingosine, and to a much lesser extent on a 20-carbon analog, phytosphingosine. All but one generic member of this class have a simple or complex sugar linked to the alcohol on carbon 1. The single deviant member is sphingomyelin, a molecule with a phosphorylcholine group (the same polar head group as in phosphatidylcholine) instead of the sugar moiety, making it an analog of phosphatidylcholine. All sphingolipids have, in addition to the sugar, a fatty acid attached to the amino group of sphingosine. Among the sphingolipids, only sphingomyelin, a phospholipid, is a major component of biological membranes. |
| |
|
|
| |
|
| General structural formula of a sphingolipid. The composition of the specific molecule depends on the chemical group (designated R2 in the diagram) linked to the alcohol “head” and also on the length of the fatty acid “tail” (R1). |
|
|
| |
| The principal factor determining the physical properties of sphingolipids is the substituent group attached to carbon 1 of sphingosine. Minor variations in properties depend upon the particular fatty acid component. The glycosphingolipids, all containing a sugar attached to carbon 1 of sphingosine, have physical properties that depend primarily on the complexity and composition of this substituent. Two generic types of glycosphingolipids are recognized: neutral glycosphingolipids, which contain only neutral sugars, and gangliosides, which contain one or more sialic acid residues linked to the sugar. Many hundreds of different glycosphingolipids have been isolated, and many more unidentified types probably exist. Glycosphingolipids are found exclusively on the external surface of the cell membrane, where their sugar moieties often act as antigens and as receptors for hormones and other signaling molecules. |

|
|
|
|
| |
Cholesterol And Its Derivatives
|
Cholesterol And Its Derivatives
Cholesterol And Its Derivatives (B)
Cholesterol may be the most intensely studied small molecule of biological origin. Not only are its complex biosynthetic pathway and the physiologically important products derived from it of scientific interest, but also the strong correlation in humans between high blood cholesterol levels and the incidence of heart attack and stroke (diseases that are leading causes of death worldwide) is of paramount medical importance. The study of this molecule and its biological origin have resulted in more than a dozen Nobel Prizes.
Cholesterol is a prominent member of a large class of lipids called isoprenoids that are widely distributed in nature. The class name derives from the fact that these molecules are formed by chemical condensation of a simple five-carbon molecule, isoprene. Isoprenoids encompass diverse biological molecules such as steroid hormones, sterols (cholesterol, ergosterol, and sitosterol), bile acids, the lipid-soluble vitamins (A, D, E, and K), phytol (a lipid component of the photosynthetic pigment chlorophyll), the insect juvenile hormones, plant hormones (gibberellins), and polyisoprene (the major component of natural rubber). Many other biologically important isoprenoids play more-subtle roles in biology. |

|
|
|
|
Structure and properties
Structure and properties (B)
The sterols are major components of biological membranes in eukaryotes (organisms whose cells have a nucleus) but are rare in prokaryotes (cells without a nucleus, such as bacteria). Cholesterol is the principal sterol of animals, whereas the major sterol in fungi is ergosterol and that in plants is sitosterol. The characteristic feature of each of these three important molecules is four rigidly fused carbon rings forming the steroid nucleus and a hydroxyl (OH) group attached to the first ring. One molecule is distinguished from another by the positions of the carbon-carbon double bonds and by the structure of the hydrocarbon side chain on the fourth ring. |
| |
|
|
| |
|
| Structural formula of cholesterol. |
|
|
| |
| Cholesterol and its relatives are hydrophobic molecules with exceedingly low water solubility. The overall hydrophobicity is negligibly affected by the hydrophilic OH group. The structure of cholesterol is such that it does not form aggregates in water, although it does shoehorn between the molecules of biological membranes, with its OH group located at the water-membrane interface. The stiff fused ring structure of cholesterol adds rigidity to liquid-crystalline phospholipid bilayers and strengthens them against mechanical rupture. Cholesterol is thus an important component of the membrane surrounding a cell, where its concentration may rise as high as 50 percent by weight. |

|
|
|
|
Biosynthesis
Biosynthesis (B)
Cholesterol biosynthesis can be divided into four stages. The first stage generates a six-carbon compound called mevalonic acid from three two-carbon acetate units (derived from the oxidation of fuel molecules—e.g., glucose) in the form of acetyl-CoA, the same initial building block used to form biological fatty acids described in the section Fatty acids: Biosynthesis. In the second stage mevalonate is converted to a five-carbon molecule of isopentenyl pyrophosphate in a series of four reactions. The conversion of this product to a 30-carbon compound, squalene, in the third stage requires the condensation of six molecules of isopentenyl pyrophosphate. In the fourth stage the linear squalene molecule is formed into rings in a complex reaction sequence to give the 27-carbon cholesterol. |
|
|
|
Biosynthetic derivatives
Biosynthetic derivatives (B)
Two classes of important molecules, bile acids and steroid hormones, are derived from cholesterol in vertebrates. These derivatives are described below. |
|
|
|
Bile acids
Bile acids (B)
The bile acids and their salts are detergents that emulsify fats in the gut during digestion. They are synthesized from cholesterol in the liver by a series of reactions that introduce a hydroxyl group into ring B and ring C and shorten the acyl side chain of ring D to seven carbons with the terminal carbon changed to a carboxyl group. The resulting molecule, cholic acid — as well as chenodeoxycholic acid (a close relative lacking the OH on ring C) — are usually found in the form of their salts, in which the amino acids taurine and glycine are chemically linked to the side-chain carboxyl group. These detergents are secreted from the liver into the gall bladder, where they are stored before being released through the bile duct into the small intestine. After performing an emulsifying action that is essential in fat digestion (described in the section Fatty acids), they are reabsorbed in the lower small intestine, returned through the blood to the liver, and reused. This cyclic process, called the enterohepatic circulation, handles 20 to 30 grams of bile acids per day in human beings. The small fraction that escapes this circulation is lost in the feces. This is the major excretory route for cholesterol (though a smaller fraction is lost through the normal sloughing of dead skin cells). |

|
|
|
|
Steroid hormones
Steroid hormones (B)
The steroid hormones consume a very small fraction of the total cholesterol available in the organism, but they are very important physiologically. (See below Biological functions of lipids.) There are five principal classes, all derived from cholesterol: progestins (active during pregnancy), the glucocorticoids (promoting the synthesis of glucose and suppressing inflammatory reactions), the mineralocorticoids (regulating ion balances), estrogens (promoting female sex characteristics), and androgens (promoting male sex characteristics). With the exception of progesterone, all of these closely related biologically active molecules have in common a shortened side chain in ring D and, in some cases, an oxidized OH group on ring A. The individual molecules are synthesized on demand by the placenta in pregnant women, by the adrenal cortex, and by the gonads. |
|
|
|
Regulation of cholesterol metabolism
Regulation of cholesterol metabolism (B)
High blood levels of cholesterol have been recognized as a primary risk factor for heart disease. For this reason, much research has been focused on the control of cholesterol’s biosynthesis, on its transport in the blood, and on its storage in the body. The overall level of cholesterol in the body is the result of a balance between dietary intake and cellular biosynthesis on the one hand and, on the other hand, elimination of cholesterol from the body (principally as its metabolic products, bile acids).
As the dietary intake of cholesterol increases in normal persons, there is a corresponding decrease in absorption from the intestines and an increase in the synthesis and excretion of bile acids—which normally accounts for about 70 percent of the cholesterol lost from the body. The molecular details of these control processes are poorly understood.
Regulation of cholesterol biosynthesis in the liver and other cells of the body is better understood. The initial enzyme that forms mevalonate in the first stage of biosynthesis is controlled by two processes. One is inhibition of the synthesis of this enzyme by cholesterol itself or a derivative of it. The other is regulation of the catalytic activity of the enzyme by phosphorylation/dephosphorylation in response to intracellular signals. Several pharmacological agents also inhibit the enzyme, with the result that unhealthy levels of cholesterol can be lowered over a period of time. |

|
|
|
|
Transport and storage
Transport and storage (B)
The normal human body contains about 100 grams of cholesterol, although this amount can vary considerably among healthy people. Approximately 60 grams of this total are moving dynamically through the organism. Because cholesterol is insoluble in water, the basis of the bodily fluids, it is carried through the circulatory system by transport particles in the blood called lipoproteins. These microscopic complexes (described in the section Lipoproteins) contain both lipids and proteins that can accommodate cholesterol and still remain soluble in blood.
Cholesterol is absorbed into the cells of the intestinal lining, where it is incorporated into lipoprotein complexes called chylomicrons and then secreted into the lymphatic circulation. The lymph ultimately enters the bloodstream, and the lipoproteins are carried to the liver. Cholesterol, whether derived from the diet or newly synthesized by the liver, is transported in the blood in lipoproteins (VLDL and LDL) to the tissues and organs of the body. There the cholesterol is incorporated into biological membranes or stored as cholesteryl esters—molecules formed by the reaction of a fatty acid (most commonly oleate) with the hydroxyl group of cholesterol. Esters of cholesterol are even more hydrophobic than cholesterol itself, and in cells they coalesce into droplets analogous to the fat droplets in adipose cells.
Cholesterol is lost from cells in peripheral tissues by transfer to another type of circulating lipoprotein (HDL) in the blood and is then returned to the liver, where it is metabolized to bile acids and salts. |

|
|
|
|
Lipoproteins
Lipoproteins (B)
Lipoproteins are lipid-protein complexes that allow all lipids derived from food or synthesized in specific organs to be transported throughout the body by the circulatory system. The basic structure of these aggregates is that of an oil droplet made up of triglycerides and cholesteryl esters surrounded by a layer of proteins and amphipathic lipids—very similar to that of a micelle, a spherical structure described in the section Fatty acids. If the concentration of one or another lipoprotein becomes too high, then a fraction of the complex becomes insoluble and is deposited on the walls of arteries and capillaries. This buildup of deposits is called atherosclerosis and ultimately results in blockage of critical arteries to cause a heart attack or stroke. Because of the gravity of this condition, much research is focused on lipoproteins and their functions. The emphasis in the following discussion is therefore placed on human lipoproteins. |
| |
|
|
| |
|
Low-density lipoprotein (LDL) complex
The LDL complex is essentially a droplet of triacylglycerols and cholesteryl esters encased in a sphere made up of phospholipid, free cholesterol, and protein molecules known as apoprotein B-100 (ApoB-100). The LDL complex is the principal vehicle for delivering cholesterol to body tissues through the blood. |
|
|

|
|
|
|
Classification and formation
Classification and formation (B)
There are four major classes of circulating lipoproteins, each with its own characteristic protein and lipid composition. They are chylomicrons, very low-density lipoproteins (VLDL), low-density lipoproteins (LDL), and high-density lipoproteins (HDL). Within all these classes of complexes, the various molecular components are not chemically linked to each other but are simply associated in such a way as to minimize hydrophobic contacts with water. The most distinguishing feature of each class is the relative amounts of lipid and protein. Because the lipid and protein composition is reflected in the density of each lipoprotein (lipid molecules being less dense than proteins), density, an easily measured attribute, forms the operational basis of defining the lipoprotein classes. Measuring density also provides the basis of separating and purifying lipoproteins from plasma for study and diagnosis. The table gives a summary of the characteristics of the lipoprotein classes and shows the correlation between composition and density. |
| |
Human plasma lipoproteins
| Source: From Christopher K. Mathews, K.E. van Holde, and Kevin G. Ahern, Biochemistry, 3rd ed. (2000), Table 18.1. |
|
chylomicron |
VLDL |
IDL |
LDL |
HDL |
| Density (g/ml) |
<0.95 |
0.950–1.006 |
1.006–1.019 |
1.019–1.063 |
1.063–1.210 |
| Components (% dry weight) |
| protein |
2 |
7 |
15 |
20 |
40–55 |
| triglycerides |
83 |
50 |
31 |
10 |
8 |
| free cholesterol |
2 |
7 |
7 |
8 |
4 |
| cholesteryl esters |
3 |
12 |
23 |
42 |
12–20 |
| phospholipids |
7 |
20 |
22 |
22 |
22 |
| Apoprotein composition |
A-I, A-II,
B-48, C-I,
C-II, C-III |
B-100, C-I,
C-II, C-III,
E |
B-100, C-I,
C-II, C-III,
E |
B-100 |
A-I, A-II,
C-I, C-II,
C-III, D, E |
|
| |
| The principal lipid components are triglycerides, cholesterol, cholesteryl esters, and phospholipids. The hydrophobic core of the particle is formed by the triglycerides and cholesteryl esters. The fatty acyl chains of these components are unsaturated, and so the core structure is liquid at body temperature. The table gives more details about the nine different protein components, called apoproteins, of the lipoprotein classes. With the exception of LDL, which contains only one type of apoprotein, all classes have multiple apoprotein components. All the apoproteins, like phospholipids, are amphipathic and interact favourably with both lipids and water. A more-detailed consideration of the character and functions of these lipoprotein particles follows. |
| |
Human plasma apolipoproteins
| Source: From Dennis E. Vance and Jean E. Vance, Biochemistry of Lipids and Membranes (1985), Table 13.4. |
| apolipoprotein |
molecular weight |
|
lipoprotein distribution |
| apoA-I |
28,331 |
|
HDL |
| apoA-II |
17,380 |
|
HDL |
| apoB-48 |
241,000 |
|
chylomicrons |
| apoB-100 |
500,000 |
|
VLDL, LDL |
| apoC-I |
7,000 |
|
HDL, VLDL |
| apoC-II |
8,837 |
|
chylomicrons, VLDL, HDL |
| apoC-III |
8,750 |
|
chylomicrons, VLDL, HDL |
| apoD |
33,000 |
|
HDL |
| apoE |
34,145 |
|
chylomicrons, VLDL, HDL |
|
| |
|
|
| |
|
| Synthesis of lipoprotein complexes in the small intestine, liver, and blood plasma and their delivery to peripheral tissues of the body. |
|
|
| |

|
|
|
|
Chylomicrons
Chylomicrons (B)
Chylomicrons are the largest lipoproteins, with diameters of 75–600 nanometres (nm; 1 nm = 10−9 metre). They have the lowest protein-to-lipid ratio (being about 90 percent lipid) and therefore the lowest density. Chylomicrons are synthesized by the absorptive cells of the intestinal lining and are secreted by these cells into the lymphatic system, which joins the blood circulation at the subclavian vein. The triglyceride, cholesteryl ester, and free cholesterol content of these particles is derived from the digestion of dietary fat. Their primary destinations in peripheral areas are heart muscle, skeletal muscle, adipose tissue, and lactating mammary tissue. The transfer of triglycerides and cholesteryl esters to the tissues depletes the lipid-protein aggregates of these substances and leaves remnant chylomicrons, which are eventually taken up by the liver. The lipid and protein remnants are used to form VLDL and LDL, described below. |
|
|
|
Very low-density lipoproteins (VLDL)
Very low-density lipoproteins (VLDL) (B)
VLDL is a lipoprotein class synthesized by the liver that is analogous to the chylomicrons secreted by the intestine. Its purpose is also to deliver triglycerides, cholesteryl esters, and cholesterol to peripheral tissues. VLDL is largely depleted of its triglyceride content in these tissues and gives rise to an intermediate-density lipoprotein (IDL) remnant, which is returned to the liver in the bloodstream. As might be expected (see table), the same proteins are present in both VLDL and IDL. |
|
|
|
Low-density lipoproteins (LDL)
Low-density lipoproteins (LDL) (B)
Low-density lipoproteins are derived from VLDL and IDL in the plasma and contain a large amount of cholesterol and cholesteryl esters. Their principal role is to deliver these two forms of cholesterol to peripheral tissues. Almost two-thirds of the cholesterol and its esters found in plasma (blood free of red and white cells) is associated with LDL. |
|
|
|
High-density lipoproteins (HDL)
High-density lipoproteins (HDL) (B)
Lipoproteins of this class are the smallest, with a diameter of 10.8 nm and the highest protein-to-lipid ratio. The resulting high density gives this class its name. HDL plays a primary role in the removal of excess cholesterol from cells and returning it to the liver, where it is metabolized to bile acids and salts that are eventually eliminated through the intestine. LDL and HDL together are the major factors in maintaining the cholesterol balance of the body. Because of the high correlation between blood cholesterol levels and atherosclerosis, high ratios of HDL to cholesterol (principally as found in LDL) correlate well with a lower incidence of this disease in humans. |
|
|
|
Functions, origins, and recycling of apolipoproteins
Functions, origins, and recycling of apolipoproteins (B)
The nine classes of apoproteins listed in the table are synthesized in the mucosal cells of the intestine and in the liver, with the liver accounting for about 80 percent of production.
Chylomicrons are synthesized in the intestinal mucosa. The cells of this tissue, although able to make most apoproteins, are the principal source of apoB (the B-48 form) and apoA-I. The apoC-II component of chylomicrons is an activator for a plasma enzyme that hydrolyzes the triglyceride of these complexes. This enzyme, called lipoprotein lipase, resides on the cell surface and makes the fatty acids of triglycerides available to the cell for energy metabolism. To some degree, the enzyme is also activated by apoC-II, present in minor amounts in chylomicrons.
VLDL, the lipoprotein carrier for triglycerides synthesized in the liver and destined for use in the heart and muscle, has a complement of five apoproteins. Among them are apoB-100, a protein performing a structural role in the complex, and apoC-I, -II, and -III. The first two of these activate the enzymes lecithin cholesterol acyltransferase (LCAT) and lipoprotein lipase. Curiously, apoC-III, a minor component of both chylomicrons and VLDL, inhibits lipoprotein lipase. Following discharge of the triglycerides, the remnants of VLDL return to the liver.
LDL contains a single apoprotein and is the principal carrier of cholesterol to the peripheral tissue as both the free sterol and esters. The discharge of the lipid contents of this complex requires the recognition of the LDL B-100 apoprotein by a receptor located on the surface of recipient cells. When the protein is bound to the receptor, the receptor-LDL complex is engulfed by the cell in a process known as endocytosis. The endocytosed LDL discharges its contents within the cell, and B-100 is degraded to free amino acids that are used to synthesize new proteins or are metabolized as an energy source. The elucidation of the process of cellular uptake of LDL by Michael Brown and Joseph Goldstein earned them the Nobel Prize for Physiology or Medicine in 1985.
The primary function of HDL with its complement of apoproteins is to take up cholesterol from the cells of the body and deliver it to the liver for its ultimate excretion as bile acids and salts. The major apoproteins are A-I, an LCAT activator, and A-II. All of the HDL apoproteins have their biosynthetic origin in the liver. When HDL is secreted by this organ, it is a small, flattened discoid devoid of cholesterol but containing phospholipids and the apoproteins. In the peripheral tissues, HDL picks up cholesterol from the surface membranes of cells and, through the agency LCAT, converts it into esters using acyl chains from phosphatidylcholine. |

|
|
|
|
| |
Biological Functions Of Lipids
|
Biological Functions Of Lipids
Biological Functions Of Lipids (B)
The majority of lipids in biological systems function either as a source of stored metabolic energy or as structural matrices and permeability barriers in biological membranes. Very small amounts of special lipids act as both intracellular messengers and extracellular messengers such as hormones and pheromones. Amphipathic lipids, the molecules that allow membranes to form compartments, must have been among the progenitors of living beings. This theory is supported by studies of several simple, single-cell organisms, in which up to one-third of the genome is thought to code for membrane proteins and the enzymes of membrane lipid biosynthesis. |
|
|
|
Cellular energy source
Cellular energy source (B)
Fatty acids that are stored in adipose tissue as triglycerides are a major energy source in higher animals, as is glucose, a simple six-carbon carbohydrate. In healthy, well-fed humans only about 2 percent of the energy is derived from the metabolism of protein. Large amounts of lipids are stored in adipose tissue. In the average American male about 25 percent of body weight is fat, whereas only 1 percent is accounted for by glycogen (a polymer of glucose). In addition, the energy available to the body from oxidative metabolism of 1 gram of triglyceride is more than twice that produced by the oxidation of an equal weight of carbohydrate such as glycogen. |
|
|
|
Storage of triglyceride in adipose cells
Storage of triglyceride in adipose cells (B)
In higher animals and humans, adipose tissue consisting of adipocytes (fat cells) is widely distributed over the body—mainly under the skin, around deep blood vessels, and in the abdominal cavity and to a lesser degree in association with muscles. Bony fishes have adipose tissue mainly distributed among muscle fibres, but sharks and other cartilaginous fishes store lipids in the liver. The fat stored in adipose tissue arises from the dietary intake of fat or carbohydrate in excess of the energy requirements of the body. A dietary excess of 1 gram of triglyceride is stored as 1 gram of fat, but only about 0.3 gram of dietary excess carbohydrate can be stored as triglyceride. The reverse process, the conversion of excess fat to carbohydrate, is metabolically impossible. In humans, excessive dietary intake can make adipose tissue the largest mass in the body.
Excess triglyceride is delivered to the adipose tissue by lipoproteins in the blood. There the triglycerides are hydrolyzed to free fatty acids and glycerol through the action of the enzyme lipoprotein lipase, which is bound to the external surface of adipose cells. Apoprotein C-II activates this enzyme, as do the quantities of insulin that circulate in the blood following ingestion of food. The liberated free fatty acids are then taken up by the adipose cells and resynthesized into triglycerides, which accumulate in a fat droplet in each cell. |

|
|
|
|
Mobilization of fatty acids
Mobilization of fatty acids (B)
In times of stress when the body requires energy, fatty acids are released from adipose cells and mobilized for use. The process begins when levels of glucagon and adrenaline in the blood increase and these hormones bind to specific receptors on the surface of adipose cells. This binding action starts a cascade of reactions in the cell that results in the activation of yet another lipase that hydrolyzes triglyceride in the fat droplet to produce free fatty acids. These fatty acids are released into the circulatory system and delivered to skeletal and heart muscle as well as to the liver. In the blood the fatty acids are bound to a protein called serum albumin; in muscle tissue they are taken up by the cells and oxidized to carbon dioxide (CO2 ) and water to produce energy, as described below. It is not clear whether a special transport mechanism is required for enabling free fatty acids to enter cells from the circulation. |
| |
|
|
| |
|
Hormone signaling; adipose tissue
When hormones signal the need for energy, fatty acids and glycerol are released from triglycerides stored in fat cells (adipocytes) and are delivered to organs and tissues in the body. |
|
|
| |
| The liver takes up a large fraction of the fatty acids. There they are in part resynthesized into triglycerides and are transported in VLDL lipoproteins to muscle and other tissues. A fraction is also converted to small ketone molecules that are exported via the circulation to peripheral tissues, where they are metabolized to yield energy. |

|
|
|
|
Oxidation of fatty acids
Oxidation of fatty acids (B)
Inside the muscle cell, free fatty acids are converted to a thioester of a molecule called coenzyme A, or CoA. (A thioester is a compound in which the linking oxygen in an ester is replaced by a sulfur atom.) Oxidation of the fatty acid–CoA thioesters actually takes place in discrete vesicular bodies called mitochondria. Most cells contain many mitochondria, each roughly the size of a bacterium, ranging from 0.5 to 10 m (micrometre; 1 m = one-millionth of a metre) in diameter; their size and shape differ depending on the cell type in which they occur. The mitochondrion is surrounded by a double membrane system enclosing a fluid interior space called the matrix. In the matrix are found the enzymes that convert the fatty acid–CoA thioesters into CO2 and water (the chemical waste products of oxidation) and also adenosine triphosphate (ATP), the energy currency of living systems. The process consists of four sequential steps.
The first step is the transport of the fatty acid across the innermost of the two concentric mitochondrial membranes. The outer membrane is very porous so that the CoA thioesters freely permeate through it. The impermeable inner membrane is a different matter; here the fatty acid chains are transported across in the following way. On the cytoplasmic side of the membrane, an enzyme catalyzes the transfer of the fatty acid from CoA to a molecule of carnitine, a hydroxy amino acid. The carnitine ester is transported across the membrane by a transferase protein located in the membrane, and on the matrix side a second enzyme catalyzes the transfer of the fatty acid from carnitine back to CoA. The carnitine that is re-formed by loss of the attached fatty acid is transferred back to the cytoplasmic side of the mitochondrial membrane to be reused. The transfer of a fatty acid from the cytoplasm to the mitochondrial matrix thus occurs without the transfer of CoA itself from one compartment to the other. No energy is generated or consumed in this transport process, although energy is required for the initial formation of the fatty acid–CoA thioester in the cytoplasm.
The second step is the oxidation of the fatty acid to a set of two-carbon acetate fragments with thioester linkages to CoA. This series of reactions, known as β-oxidation, takes place in the matrix of the mitochondrion. Since most biological fatty acids have an even number of carbons, the number of acetyl-CoA fragments derived from a specific fatty acid is equal to one-half the number of carbons in the acyl chain. For example, palmitic acid (C16) yields eight acetyl-CoA thioesters. In the case of rare unbranched fatty acids with an odd number of carbons, one three-carbon CoA ester is formed as well as the two-carbon acetyl-CoA thioesters. Thus, a C17 acid yields seven acetyl and one three-carbon CoA thioester. The energy in the successive oxidation steps is conserved by chemical reduction (the opposite of oxidation) of molecules that can subsequently be used to form ATP. ATP is the common fuel used in all the machinery of the cell (e.g., muscle, nerves, membrane transport systems, and biosynthetic systems for the formation of complex molecules such as DNA and proteins).
The two-carbon residues of acetyl-CoA are oxidized to CO2 and water, with conservation of chemical energy in the form of FADH2 and NADH and a small amount of ATP. This process is carried out in a series of nine enzymatically catalyzed reactions in the mitochondrial matrix space. The reactions form a closed cycle, often called the citric acid, tricarboxylic acid, or Krebs cycle (after its discoverer, Nobelist Sir Hans Krebs).
The final stage is the conversion of the chemical energy in NADH and FADH2 formed in the second and third steps into ATP by a process known as oxidative phosphorylation. All the participating enzymes are located inside the mitochondrial inner membrane—except one, which is trapped in the space between the inner and outer membranes. In order for the process to produce ATP, the inner membrane must be impermeable to hydrogen ions (H+). In the course of oxidative phosphorylation, molecules of NADH and FADH2 are subjected to a series of linked oxidation-reduction reactions. NADH and FADH2 are rich in electrons and give up these electrons to the first member of the reaction chain. The electrons then pass down the series of oxidation-reduction reactions and in the last reaction reduce molecular oxygen (O2) to water (H2O). This part of oxidative phosphorylation is called electron transport.
The chemical energy available in these electron-transfer reactions is conserved by pumping H+ across the mitochondrial inner membrane from matrix to cytoplasm. Essentially an electrical battery is created, with the cytoplasm acting as the positive pole and the mitochondrial matrix as the negative pole. The net effect of electron transport is thus to convert the chemical energy of oxidation into the electrical energy of the transmembrane “battery.” The energy stored in this battery is in turn used to generate ATP from adenosine diphosphate (ADP) and inorganic phosphate by the action of a complex enzyme called ATP synthase, also located on the inner mitochondrial membrane. Peter Mitchell received the Nobel Prize for Chemistry in 1978 for his discovery of the conversion of electron transport energy into a transmembrane battery and the use of this battery to generate ATP. It is interesting that a similar process forms the basis of photosynthesis—the mechanism by which green plants convert light energy from the Sun into carbohydrates and fats, the basic foods of both plants and animals. Many of the molecular details of the oxidative phosphorylation system are now known, but there is still much to learn about it and the equally complex process of photosynthesis.
The β-oxidation also occurs to a minor extent within small subcellular organelles called peroxisomes in animals and glyoxysomes in plants. In these cases fatty acids are oxidized to CO2 and water, but the energy is released as heat. The biochemical details and physiological functions of these organelles are not well understood. |

|
|
|
|
Regulation of fatty acid oxidation
Regulation of fatty acid oxidation (B)
The rate of utilization of acetyl-CoA, the product of β-oxidation, and the availability of free fatty acids are the determining factors that control fatty acid oxidation. The concentrations of free fatty acids in the blood are hormone-regulated, with glucagon stimulating and insulin inhibiting fatty acid release from adipose tissue. The utilization in muscle of acetyl-CoA depends upon the activity of the citric acid cycle and oxidative phosphorylation—whose rates in turn reflect the demand for ATP.
In the liver the metabolism of free fatty acids reflects the metabolic state of the animal. In well-fed animals the liver converts excess carbohydrates to fatty acids, whereas in fasting animals fatty acid oxidation is the predominant activity, along with the formation of ketones. Although the details are not completely understood, it is clear that in the liver the metabolism of fatty acids is tightly linked to fatty acid synthesis so that a wasteful closed cycle of fatty acid synthesis from and metabolism back to acetyl-CoA is prevented. |

|
|
|
|
Lipids in biological membranes
Lipids in biological membranes (B)
Biological membranes separate the cell from its environment and compartmentalize the cell interior. The various membranes playing these vital roles are composed of roughly equal weight percent protein and lipid, with carbohydrates constituting less than 10 percent in a few membranes. Although many hundreds of molecular species are present in any one membrane, the general organization of the generic components is known. All the lipids are amphipathic, with their hydrophilic (polar) and hydrophobic (nonpolar) portions located at separate parts of each molecule. As a result, the lipid components of membranes are arranged in what may be called a continuous bimolecular leaflet, or bilayer. The polar portions of the constituent molecules lie in the two bilayer faces, while the nonpolar portions constitute the interior of the bilayer. The lipid bilayer structure forms an impermeable barrier for essential water-soluble substances in the cell and provides the basis for the compartmentalizing function of biological membranes. |
| |
|
|
| |
|
Molecular view of the cell membrane
Intrinsic proteins penetrate and bind tightly to the lipid bilayer, which is made up largely of phospholipids and cholesterol and which typically is between 4 and 10 nanometers (nm; 1 nm = 10−9 metre) in thickness. Extrinsic proteins are loosely bound to the hydrophilic (polar) surfaces, which face the watery medium both inside and outside the cell. Some intrinsic proteins present sugar side chains on the cell's outer surface. |
|
|
Some protein components are inserted into the bilayer, and most span this structure. These so-called integral, or intrinsic, membrane proteins have amino acids with nonpolar side chains at the interface between the protein and the nonpolar central region of the lipid bilayer. A second class of proteins is associated with the polar surfaces of the bilayer and with the intrinsic membrane proteins. The protein components are specific for each type of membrane and determine their predominant physiological functions. The lipid component, apart from its critical barrier function, is for the most part physiologically silent, although derivatives of certain membrane lipids can serve as intracellular messengers.
The most remarkable feature of the general biomembrane structure is that the lipid and the protein components are not covalently bonded to one another or to molecules of the other group. This sheetlike structure, formed only by molecular associations, is less than 10 nm in thickness but many orders of magnitude larger in its other two dimensions. Membranes are surprisingly strong mechanically, yet they exhibit fluidlike properties. Although the surfaces of membranes contain polar units, they act as an electric insulator and can withstand several hundred thousand volts without breakdown. Experimental and theoretical studies have established that the structure and these unusual properties are conferred on biological membranes by the lipid bilayer. |

|
|
|
|
Composition of the lipid bilayer
Composition of the lipid bilayer (B)
Most biological membranes contain a variety of lipids, including the various glycerophospholipids such as phosphatidyl-choline, -ethanolamine, -serine, -inositol, and -glycerol as well as sphingomyelin and, in some membranes, glycosphingolipids. (These compounds are described in the section Fatty acid derivatives.) Cholesterol, ergosterol, and sitosterol (described in the section Cholesterol and its derivatives) are sterols found in many membranes. The relative amounts of these lipids differ even in the same type of cell in different organisms, as shown in the table on the lipid composition of red blood cell membranes from different mammalian species. Even in a single cell, the lipid compositions of the membrane surrounding the cell (the plasma membrane) and the membranes of the various organelles within the cell (such as the microsomes, mitochondria, and nucleus) are different, as shown in the table on various membranes in a rat liver cell. |
| |
Organelle membrane lipid composition by weight percent of rat liver cells
| Source: From Thomas E. Andreoli et al., Membrane Physiology, 2nd ed. (1987), Table II, chapter 27. |
|
membrane |
| lipid |
plasma membrane |
microsome |
inner mitochondria |
outer
mitochondria |
nuclear |
| cholesterol |
28.0 |
6.0 |
<1.0 |
6.0 |
5.1 |
| phosphatidylcholine |
31.0 |
55.20 |
37.9 |
42.70 |
58.30 |
| sphingomyelin |
16.6 |
3.7 |
00.8 |
4.1 |
3.0 |
| phosphatidylethanolamine |
14.3 |
24.00 |
38.3 |
28.60 |
21.50 |
| phosphatidylserine |
02.7 |
— |
<1.0 |
<1.00 |
3.4 |
| phosphatidylinositol |
04.7 |
7.7 |
02.0 |
7.9 |
8.2 |
| phosphatidic acid and cardiolipin |
01.4 |
1.5 |
20.4 |
8.9 |
<1.00 |
| lysophosphatidylcholine |
01.3 |
1.9 |
00.6 |
1.7 |
|
| |
Plasma membrane lipid composition by weight percent of mammalian red blood cells
| Source: From Thomas E. Andreoli et al., Membrane Physiology, 2nd ed. (1987), Table I, chapter 27. |
|
species |
| lipid |
pig |
human |
cat |
rabbit |
horse |
rat |
| cholesterol |
26.8 |
26.0 |
26.8 |
28.9 |
24.5 |
24.7 |
| phosphatidylcholine |
13.9 |
17.5 |
18.7 |
22.3 |
22.0 |
31.8 |
| sphingomyelin |
15.8 |
16.0 |
16.0 |
12.5 |
07.0 |
08.6 |
| phosphatidylethanolamine |
17.7 |
16.6 |
13.6 |
21.0 |
12.6 |
14.4 |
| phosphatidylserine |
10.6 |
07.9 |
08.1 |
08.0 |
09.4 |
07.2 |
| phosphatidylinositol |
01.1 |
01.2 |
04.5 |
01.0 |
<0.2 |
02.3 |
| phosphatidic acid |
<0.2 |
00.6 |
00.5 |
01.0 |
<0.2 |
<0.2 |
| lysophosphatidylcholine |
00.5 |
00.9 |
<0.2 |
<0.2 |
00.9 |
02.6 |
| glycosphingolipids |
13.4 |
11.0 |
11.9 |
05.3 |
23.5 |
08.3 |
|
| |

|
|
|
|
Physical characteristics of membranes
Physical characteristics of membranes (B)
One of the most surprising characteristics of biological membranes is the fact that both the lipid and the protein molecules, like molecules in any viscous liquid, are constantly in motion. Indeed, the membrane can be considered a two-dimensional liquid in which the protein components ride like boats. However, the lipid molecules in the bilayer must always be oriented with their polar ends at the surface and their nonpolar parts in the central region of the bilayer. The bilayer structure thus has the molecular orientation of a crystal and the fluidity of a liquid. In this liquid-crystalline state, thermal energy causes both lipid and protein molecules to diffuse laterally and also to rotate about an axis perpendicular to the membrane plane. In addition, the lipids occasionally flip from one face of the membrane bilayer to the other and attach and detach from the surface of the bilayer at very slow but measurable rates. Although these latter motions are forbidden to intrinsic proteins, both lipids and proteins can exhibit limited bobbing motions. Within this seemingly random, dynamic mixture of components, however, there is considerable order in the plane of the membrane. This order takes the form of a “fluid mosaic” of molecular association complexes of both lipids and proteins in the membrane plane. The plane of the biological membrane is thus compartmentalized by domain structures much as the three-dimensional space of the cell is compartmentalized by the membranes themselves. The domain mosaics run in size from tens of nanometres (billionths of a metre) to micrometres (millionths of a metre) and are stable over time intervals of nanoseconds to minutes. In addition to this in-plane domain structure, the two lipid monolayers making up the membrane bilayer frequently have different compositions. This asymmetry, combined with the fact that intrinsic membrane proteins do not rotate about an axis in the membrane plane, makes the two halves of the bilayer into separate domains.
An interesting class of proteins is attached to biological membranes by a lipid that is chemically linked to the protein. Many of these proteins are involved in intra- and intercellular signaling. In some cases defects in their structure render the cells cancerous, presumably because growth-limiting signals are blocked by the structural error. |

|
|
|
|
Intracellular and extracellular messengers
Intracellular and extracellular messengers (B)
Intracellular and extracellular messengers
In multicellular organisms (eukaryotes), the internal mechanisms that control and coordinate basic biochemical reactions are connected to other cells by means of nerves and chemical “messengers.” The overall process of receiving these messages and converting the information they contain into metabolic and physiological effects is known as signal transduction. Many of the chemical messengers are lipids and are thus of special interest here. There are several types of external messengers. The first of these are hormones such as insulin and glucagon and the lipids known collectively as steroid hormones. A second class of lipid molecules is eicosanoids, which are produced in tissues and elicit cellular responses close to their site of origin. They are produced in very low levels and are turned over very rapidly (in seconds). Hormones have sites of action that are remote from their cells of origin and remain in the circulation for long periods (minutes to hours). |

|
|
|
|
Steroid hormones
Steroid hormones (B)
Lipid hormones invoke changes in gene expression; that is, their action is to turn on or off the instructions issued by deoxyribonucleic acid (DNA) to produce proteins that regulate the biosynthesis of other important proteins. Steroids are carried in the circulation bound singly to specific carrier proteins that target them to the cells in particular organs. After permeating the external membranes of these cells, the steroid interacts with a specific carrier protein in the cytoplasm. This soluble complex migrates into the cell nucleus, where it interacts with the DNA to activate or repress transcription, the first step in protein biosynthesis.
All five major classes of steroid hormones produced from cholesterol contain the characteristic five rings of carbon atoms of the parent molecule. Progestins are a group of steroids that regulate events during pregnancy and are the precursors of the other steroid hormones. The glucocorticoids, cortisol, and corticosterones promote the biosynthesis of glucose and act to suppress inflammation. The mineralocorticoids regulate ion balances between the interior and the exterior of the cell. Androgens regulate male sexual characteristics, and estrogens perform an analogous function in females. The target organs for these hormones are listed in the table. |
| |
Organs affected by steroid hormones
| Source: From Christopher K. Mathews, K.E. van Holde, and Kevin G. Ahern, Biochemistry, 3rd ed. (2000), Table 23.6. |
| hormone class |
target organs |
| glucocorticoids |
liver, retina, kidney, oviduct, pituitary |
| estrogens |
oviduct, liver |
| progesterone |
oviduct, uterus |
| androgens |
prostate, kidney, oviduct |
|

|
|
|
|
Eicosanoids
Eicosanoids (B)
Three types of locally acting signaling molecules are derived biosynthetically from C20 polyunsaturated fatty acids, principally arachidonic acid. Twenty-carbon fatty acids are all known collectively as eicosanoic acids. The three chemically similar classes are prostaglandins, thromboxanes, and leukotrienes. The eicosanoids interact with specific cell surface receptors to produce a variety of different effects on different tissues, but generally they cause inflammatory responses and changes in blood pressure, and they also affect the clotting of blood. Little is known about how these effects are produced within the cells of target tissues. However, it is known that aspirin and other anti-inflammatory drugs inhibit either an enzyme in the biosynthesis pathway or the eicosanoid receptor on the cell surface. |
|
|
|
Intracellular second messengers
Intracellular second messengers (B)
With the exception of the steroid hormones, most hormones such as insulin and glucagon interact with a receptor on the cell surface. The activated receptor then generates so-called second messengers within the cell that transmit the information to the biochemical systems whose activities must be altered to produce a particular physiological effect. The magnitude of the end effect is generally proportional to the concentration of the second messengers.
An important intracellular second-messenger signaling system, the phosphatidylinositol system, employs two second-messenger lipids, both of which are derived from phosphatidylinositol. One is diacylglycerol ( diglyceride), the other is triphosphoinositol. In this system a membrane receptor acts upon an enzyme, phospholipase C, located on the inner surface of the cell membrane. Activation of this enzyme by one of the agents listed in the table causes the hydrolysis of a minor membrane phospholipid, phosphatidylinositol bisphosphate. Without leaving the membrane bilayer, the diacylglycerol next activates a membrane-bound enzyme, protein kinase C, that in turn catalyzes the addition of phosphate groups to a soluble protein. This soluble protein is the first member of a reaction sequence leading to the appropriate physiological response in the cell. The other hydrolysis product of phospholipase C, triphosphoinositol, causes the release of calcium from intracellular stores. Calcium is required, in addition to triacylglycerol, for the activation of protein kinase C. |
| |
Tissue affected by phosphoinositide second-messenger system
Source: From Christopher K. Mathews, K.E. van Holde, and Kevin G. Ahern, Biochemistry, 3rd ed. (2000),
Table 23.5. |
| extracellular signal |
target tissue |
cellular response |
| acetylcholine |
pancreas
pancreas (islet cells)
smooth muscle |
amylase secretion
insulin release
contraction |
| vasopressin |
liver
kidney |
glycogenolysis |
| thrombin |
blood platelets |
platelet aggregation |
| antigens |
lymphoblasts
mast cells |
DNA synthesis
histamine secretion |
| growth factors |
fibroblasts |
DNA synthesis |
| spermatozoa |
eggs (sea urchin) |
fertilization |
| light |
photoreceptors (horseshoe crab) |
phototransduction |
| thyrotropin-releasing hormone |
pituitary anterior lobe |
prolactin secretion |
|

|
|
|
|

|
|
|
|
|
|
Lipid
In biology and biochemistry, a lipid is a macrobiomolecule that is soluble in nonpolar solvents. Non-polar solvents are typically hydrocarbons used to dissolve other naturally occurring hydrocarbon lipid molecules that do not (or do not easily) dissolve in water, including fatty acids, waxes, sterols, fat-soluble vitamins (such as vitamins A, D, E, and K), monoglycerides, diglycerides, triglycerides, and phospholipids.
The functions of lipids include storing energy, signaling, and acting as structural components of cell membranes. Lipids have applications in the cosmetic and food industries as well as in nanotechnology.
Scientists sometimes define lipids as hydrophobic or amphiphilic small molecules; the amphiphilic nature of some lipids allows them to form structures such as vesicles, multilamellar/unilamellar liposomes, or membranes in an aqueous environment. Biological lipids originate entirely or in part from two distinct types of biochemical subunits or "building-blocks": ketoacyl and isoprene groups. Using this approach, lipids may be divided into eight categories: fatty acids, glycerolipids, glycerophospholipids, sphingolipids, saccharolipids, and polyketides (derived from condensation of ketoacyl subunits); and sterol lipids and prenol lipids (derived from condensation of isoprene subunits).
Although the term "lipid" is sometimes used as a synonym for fats, fats are a subgroup of lipids called triglycerides. Lipids also encompass molecules such as fatty acids and their derivatives (including tri-, di-, monoglycerides, and phospholipids), as well as other sterol-containing metabolites such as cholesterol. Although humans and other mammals use various biosynthetic pathways both to break down and to synthesize lipids, some essential lipids can't be made this way and must be obtained from the diet. |
| |
|
|
| |
History
|
History
History (W)
Lipid may be regarded as organic substances relatively insoluble in water, soluble in organic solvents(alcohol, ether etc.) actually or potentially related to fatty acid and utilized by the living cells.
In 1815, Henri Braconnot classified lipids (graisses) in two categories, suifs (solid greases or tallow) and huiles (fluid oils). In 1823, Michel Eugène Chevreul developed a more detailed classification, including oils, greases, tallow, waxes, resins, balsams and volatile oils (or essential oils).
The first successful synthesis of a triglyceride molecule was by Théophile-Jules Pelouze in 1844, when he produced tributyrin by reacting butyric acid with glycerin in the presence of concentrated sulfuric acid. Several years later, Marcellin Berthelot, one of Pelouze's students, synthesized tristearin and tripalmitin by reaction of the analogous fatty acids with glycerin in the presence of gaseous hydrogen chloride at high temperature.
In 1827, William Prout recognized fat ("oily" alimentary matters), along with protein ("albuminous") and carbohydrate ("saccharine"), as an important nutrient for humans and animals.
For a century, chemists regarded "fats" as only simple lipids made of fatty acids and glycerol (glycerides), but new forms were described later. Theodore Gobley (1847) discovered phospholipids in mammalian brain and hen egg, called by him as "lecithins". Thudichum discovered in human brain some phospholipids (cephalin), glycolipids (cerebroside) and sphingolipids (sphingomyelin).
The terms lipoid, lipin, lipide and lipid have been used with varied meanings from author to author. In 1912, Rosenbloom and Gies proposed the substitution of "lipoid" by "lipin". In 1920, Bloor introduced a new classification for "lipoids": simple lipoids (greases and waxes), compound lipoids (phospholipoids and glycolipoids), and the derived lipoids (fatty acids, alcohols, sterols).
The word "lipide" , which stems etymologically from the Greek lipos (fat), was introduced in 1923 by the french pharmacologist Gabriel Bertrand. Bertrands included in the concept not only the traditional fats (glycerides), but also the "lipoids", with a complex constitution. Despite the word "lipide" was unanimously approved by the international commission of Société de Chimie Biologique during the plenary session on the 3rd of July 1923. The word "lipide" has been later anglicized as "lipid" because of its pronunciation ('lɪpɪd). In the french language, the suffixe "-ide", from the ancient greek "-ίδης" (meaning 'son of' or 'descendant of'), is always pronounced (ɪd).
In 1947, T. P. Hilditch divided lipids into "simple lipids", with greases and waxes (true waxes, sterols, alcohols). |

|
|
|
|
| |
Categories
|
Categories
Categories (W)
Lipids have been classified into eight categories by the Lipid MAPS consortium as follows: |
|
|
|
Fatty acids
Fatty acids (W)
|
|
| |
|
| I2 - Prostacyclin (an example of a prostaglandin, an eicosanoid fatty acid) |
|
| |
|
Fatty acids, or fatty acid residues when they are part of a lipid, are a diverse group of molecules synthesized by chain-elongation of an acetyl-CoA primer with malonyl-CoA or methylmalonyl-CoA groups in a process called fatty acid synthesis. They are made of a hydrocarbon chain that terminates with a carboxylic acid group; this arrangement confers the molecule with a polar, hydrophilic end, and a nonpolar, hydrophobic end that is insoluble in water. The fatty acid structure is one of the most fundamental categories of biological lipids and is commonly used as a building-block of more structurally complex lipids. The carbon chain, typically between four and 24 carbons long, may be saturated or unsaturated, and may be attached to functional groups containing oxygen, halogens, nitrogen, and sulfur. If a fatty acid contains a double bond, there is the possibility of either a cis or trans geometric isomerism, which significantly affects the molecule's configuration. Cis-double bonds cause the fatty acid chain to bend, an effect that is compounded with more double bonds in the chain. Three double bonds in 18-carbon linolenic acid, the most abundant fatty-acyl chains of plant thylakoid membranes, render these membranes highly fluid despite environmental low-temperatures, and also makes linolenic acid give dominating sharp peaks in high resolution 13-C NMR spectra of chloroplasts. This in turn plays an important role in the structure and function of cell membranes. Most naturally occurring fatty acids are of the cis configuration, although the trans form does exist in some natural and partially hydrogenated fats and oils.
Examples of biologically important fatty acids include the eicosanoids, derived primarily from arachidonic acid and eicosapentaenoic acid, that include prostaglandins, leukotrienes, and thromboxanes. Docosahexaenoic acid is also important in biological systems, particularly with respect to sight. Other major lipid classes in the fatty acid category are the fatty esters and fatty amides. Fatty esters include important biochemical intermediates such as wax esters, fatty acid thioester coenzyme A derivatives, fatty acid thioester ACP derivatives and fatty acid carnitines. The fatty amides include N-acyl ethanolamines, such as the cannabinoid neurotransmitter anandamide. |

|
|
|
|
Glycerolipids
Glycerolipids (W)
Glycerolipids are composed of mono-, di-, and tri-substituted glycerols, the best-known being the fatty acid triesters of glycerol, called triglycerides. The word "triacylglycerol" is sometimes used synonymously with "triglyceride". In these compounds, the three hydroxyl groups of glycerol are each esterified, typically by different fatty acids. Because they function as an energy store, these lipids comprise the bulk of storage fat in animal tissues. The hydrolysis of the ester bonds of triglycerides and the release of glycerol and fatty acids from adipose tissue are the initial steps in metabolizing fat.
Additional subclasses of glycerolipids are represented by glycosylglycerols, which are characterized by the presence of one or more sugar residues attached to glycerol via a glycosidic linkage. Examples of structures in this category are the digalactosyldiacylglycerols found in plant membranes and seminolipid from mammalian sperm cells. |
| |
|

|
|
|
|
Glycerophospholipids
Glycerophospholipids (W)
Glycerophospholipids, usually referred to as phospholipids (though sphingomyelins are also classified as phospholipids), are ubiquitous in nature and are key components of the lipid bilayer of cells, as well as being involved in metabolism and cell signaling. Neural tissue (including the brain) contains relatively high amounts of glycerophospholipids, and alterations in their composition has been implicated in various neurological disorders. Glycerophospholipids may be subdivided into distinct classes, based on the nature of the polar headgroup at the sn-3 position of the glycerol backbone in eukaryotes and eubacteria, or the sn-1 position in the case of archaebacteria.
Examples of glycerophospholipids found in biological membranes are phosphatidylcholine (also known as PC, GPCho or lecithin), phosphatidylethanolamine (PE or GPEtn) and phosphatidylserine (PS or GPSer). In addition to serving as a primary component of cellular membranes and binding sites for intra- and intercellular proteins, some glycerophospholipids in eukaryotic cells, such as phosphatidylinositols and phosphatidic acids are either precursors of or, themselves, membrane-derived second messengers. Typically, one or both of these hydroxyl groups are acylated with long-chain fatty acids, but there are also alkyl-linked and 1Z-alkenyl-linked (plasmalogen) glycerophospholipids, as well as dialkylether variants in archaebacteria. |
| |
|

|
|
|
|
Sphingolipids
Sphingolipids (W)
Sphingolipids are a complicated family of compounds that share a common structural feature, a sphingoid base backbone that is synthesized de novo from the amino acid serine and a long-chain fatty acyl CoA, then converted into ceramides, phosphosphingolipids, glycosphingolipids and other compounds. The major sphingoid base of mammals is commonly referred to as sphingosine. Ceramides (N-acyl-sphingoid bases) are a major subclass of sphingoid base derivatives with an amide-linked fatty acid. The fatty acids are typically saturated or mono-unsaturated with chain lengths from 16 to 26 carbon atoms.
The major phosphosphingolipids of mammals are sphingomyelins (ceramide phosphocholines), whereas insects contain mainly ceramide phosphoethanolamines and fungi have phytoceramide phosphoinositols and mannose-containing headgroups. The glycosphingolipids are a diverse family of molecules composed of one or more sugar residues linked via a glycosidic bond to the sphingoid base. Examples of these are the simple and complex glycosphingolipids such as cerebrosides and gangliosides. |
| |
|

|
|
|
|
Sterols
Sterols (W)
Sterols, such as cholesterol and its derivatives, are an important component of membrane lipids, along with the glycerophospholipids and sphingomyelins. Other examples of sterols are the bile acids and their conjugates, which in mammals are oxidized derivatives of cholesterol and are synthesized in the liver. The plant equivalents are the phytosterols, such as β-sitosterol, stigmasterol, and brassicasterol; the latter compound is also used as a biomarker for algal growth. The predominant sterol in fungal cell membranes is ergosterol.
Sterols are steroids in which one of the hydrogen atoms is substituted with a hydroxyl group, at position 3 in the carbon chain. They have in common with steroids the same fused four-ring core structure. Steroids have different biological roles as hormones and signaling molecules. The eighteen-carbon (C18) steroids include the estrogen family whereas the C19 steroids comprise the androgens such as testosterone and androsterone. The C21 subclass includes the progestogens as well as the glucocorticoids and mineralocorticoids. The secosteroids, comprising various forms of vitamin D, are characterized by cleavage of the B ring of the core structure. |
| |
|

|
|
|
|
Prenols
Prenols (W)
Prenol lipids are synthesized from the five-carbon-unit precursors isopentenyl diphosphate and dimethylallyl diphosphate that are produced mainly via the mevalonic acid (MVA) pathway. The simple isoprenoids (linear alcohols, diphosphates, etc.) are formed by the successive addition of C5 units, and are classified according to number of these terpene units. Structures containing greater than 40 carbons are known as polyterpenes. Carotenoids are important simple isoprenoids that function as antioxidants and as precursors of vitamin A. Another biologically important class of molecules is exemplified by the quinones and hydroquinones, which contain an isoprenoid tail attached to a quinonoid core of non-isoprenoid origin. Vitamin E and vitamin K, as well as the ubiquinones, are examples of this class. Prokaryotes synthesize polyprenols (called bactoprenols) in which the terminal isoprenoid unit attached to oxygen remains unsaturated, whereas in animal polyprenols (dolichols) the terminal isoprenoid is reduced. |
| |
|
|
|
| |
|
| Prenol lipid (2E-geraniol). |
|
|

|
|
|
|
Saccharolipids
Saccharolipids (W)
Saccharolipids describe compounds in which fatty acids are linked directly to a sugar backbone, forming structures that are compatible with membrane bilayers. In the saccharolipids, a monosaccharide substitutes for the glycerol backbone present in glycerolipids and glycerophospholipids. The most familiar saccharolipids are the acylated glucosamine precursors of the Lipid A component of the lipopolysaccharides in Gram-negative bacteria. Typical lipid A molecules are disaccharides of glucosamine, which are derivatized with as many as seven fatty-acyl chains. The minimal lipopolysaccharide required for growth in E. coli is Kdo2-Lipid A, a hexa-acylated disaccharide of glucosamine that is glycosylated with two 3-deoxy-D-manno-octulosonic acid (Kdo) residues. |
| |
____________. |
|
| |
| Structure of the saccharolipid Kdo2-lipid A. Glucosamine residues in blue, Kdo residues in red, acyl chains in black and phosphate groups in green |
|
|
|
|
Polyketides
Polyketides (W)
Polyketides are synthesized by polymerization of acetyl and propionyl subunits by classic enzymes as well as iterative and multimodular enzymes that share mechanistic features with the fatty acid synthases. They comprise many secondary metabolites and natural products from animal, plant, bacterial, fungal and marine sources, and have great structural diversity. Many polyketides are cyclic molecules whose backbones are often further modified by glycosylation, methylation, hydroxylation, oxidation, or other processes. Many commonly used anti-microbial, anti-parasitic, and anti-cancer agents are polyketides or polyketide derivatives, such as erythromycins, tetracyclines, avermectins, and antitumor epothilones. |
|
|
|
| |
Biological functions
|
Membranes
Membranes (W)
|
|
|
| |
|
| Self-organization of phospholipids: a spherical liposome, a micelle, and a lipid bilayer. |
|
Eukaryotic cells feature the compartmentalized membrane-bound organelles that carry out different biological functions. The glycerophospholipids are the main structural component of biological membranes, as the cellular plasma membrane and the intracellular membranes of organelles; in animal cells, the plasma membrane physically separates the intracellular components from the extracellular environment.The glycerophospholipids are amphipathic molecules (containing both hydrophobic and hydrophilic regions) that contain a glycerol core linked to two fatty acid-derived "tails" by ester linkages and to one "head" group by a phosphate ester linkage.[citation needed] While glycerophospholipids are the major component of biological membranes, other non-glyceride lipid components such as sphingomyelin and sterols (mainly cholesterol in animal cell membranes) are also found in biological membranes. In plants and algae, the galactosyldiacylglycerols, and sulfoquinovosyldiacylglycerol, which lack a phosphate group, are important components of membranes of chloroplasts and related organelles and are the most abundant lipids in photosynthetic tissues, including those of higher plants, algae and certain bacteria.
Plant thylakoid membranes have the largest lipid component of a non-bilayer forming monogalactosyl diglyceride (MGDG), and little phospholipids; despite this unique lipid composition, chloroplast thylakoid membranes have been shown to contain a dynamic lipid-bilayer matrix as revealed by magnetic resonance and electron microscope studies.
A biological membrane is a form of lamellar phase lipid bilayer. The formation of lipid bilayers is an energetically preferred process when the glycerophospholipids described above are in an aqueous environment. This is known as the hydrophobic effect. In an aqueous system, the polar heads of lipids align towards the polar, aqueous environment, while the hydrophobic tails minimize their contact with water and tend to cluster together, forming a vesicle; depending on the concentration of the lipid, this biophysical interaction may result in the formation of micelles, liposomes, or lipid bilayers. Other aggregations are also observed and form part of the polymorphism of amphiphile (lipid) behavior. Phase behavior is an area of study within biophysics and is the subject of current academic research. Micelles and bilayers form in the polar medium by a process known as the hydrophobic effect. When dissolving a lipophilic or amphiphilic substance in a polar environment, the polar molecules (i.e., water in an aqueous solution) become more ordered around the dissolved lipophilic substance, since the polar molecules cannot form hydrogen bonds to the lipophilic areas of the amphiphile. So in an aqueous environment, the water molecules form an ordered "clathrate" cage around the dissolved lipophilic molecule.
The formation of lipids into protocell membranes represents a key step in models of abiogenesis, the origin of life. |

|
|
|
|
Energy storage
Energy storage (W)
Triglycerides, stored in adipose tissue, are a major form of energy storage both in animals and plants. They are a major source of energy because carbohydrates are fully reduced structures. In comparison to glycogen which would contribute only half of the energy per its pure mass, triglyceride carbons are all bonded to hydrogens, unlike in carbohydrates. The adipocyte, or fat cell, is designed for continuous synthesis and breakdown of triglycerides in animals, with breakdown controlled mainly by the activation of hormone-sensitive enzyme lipase. The complete oxidation of fatty acids provides high caloric content, about 38 kJ/g (9 kcal/g), compared with 17 kJ/g (4 kcal/g) for the breakdown of carbohydrates and proteins. Migratory birds that must fly long distances without eating use stored energy of triglycerides to fuel their flights. |
|
|
|
Signaling
Signaling (W)
In recent years, evidence has emerged showing that lipid signaling is a vital part of the cell signaling. Lipid signaling may occur via activation of G protein-coupled or nuclear receptors, and members of several different lipid categories have been identified as signaling molecules and cellular messengers. These include sphingosine-1-phosphate, a sphingolipid derived from ceramide that is a potent messenger molecule involved in regulating calcium mobilization, cell growth, and apoptosis; diacylglycerol (DAG) and the phosphatidylinositol phosphates (PIPs), involved in calcium-mediated activation of protein kinase C; the prostaglandins, which are one type of fatty-acid derived eicosanoid involved in inflammation and immunity; the steroid hormones such as estrogen, testosterone and cortisol, which modulate a host of functions such as reproduction, metabolism and blood pressure; and the oxysterols such as 25-hydroxy-cholesterol that are liver X receptor agonists. Phosphatidylserine lipids are known to be involved in signaling for the phagocytosis of apoptotic cells or pieces of cells. They accomplish this by being exposed to the extracellular face of the cell membrane after the inactivation of flippases which place them exclusively on the cytosolic side and the activation of scramblases, which scramble the orientation of the phospholipids. After this occurs, other cells recognize the phosphatidylserines and phagocytosize the cells or cell fragments exposing them. |

|
|
|
|
Other functions
Other functions (W)
The "fat-soluble" vitamins (A, D, E and K) – which are isoprene-based lipids – are essential nutrients stored in the liver and fatty tissues, with a diverse range of functions. Acyl-carnitines are involved in the transport and metabolism of fatty acids in and out of mitochondria, where they undergo beta oxidation. Polyprenols and their phosphorylated derivatives also play important transport roles, in this case the transport of oligosaccharides across membranes. Polyprenol phosphate sugars and polyprenol diphosphate sugars function in extra-cytoplasmic glycosylation reactions, in extracellular polysaccharide biosynthesis (for instance, peptidoglycan polymerization in bacteria), and in eukaryotic protein N-glycosylation. Cardiolipins are a subclass of glycerophospholipids containing four acyl chains and three glycerol groups that are particularly abundant in the inner mitochondrial membrane. They are believed to activate enzymes involved with oxidative phosphorylation. Lipids also form the basis of steroid hormones. |

|
|
|
|
| |
Metabolism
|
Metabolism
Metabolism (W)
The major dietary lipids for humans and other animals are animal and plant triglycerides, sterols, and membrane phospholipids. The process of lipid metabolism synthesizes and degrades the lipid stores and produces the structural and functional lipids characteristic of individual tissues. |
|
|
|
Biosynthesis
Biosynthesis (W)
In animals, when there is an oversupply of dietary carbohydrate, the excess carbohydrate is converted to triglycerides. This involves the synthesis of fatty acids from acetyl-CoA and the esterification of fatty acids in the production of triglycerides, a process called lipogenesis. Fatty acids are made by fatty acid synthases that polymerize and then reduce acetyl-CoA units. The acyl chains in the fatty acids are extended by a cycle of reactions that add the acetyl group, reduce it to an alcohol, dehydrate it to an alkene group and then reduce it again to an alkane group. The enzymes of fatty acid biosynthesis are divided into two groups, in animals and fungi all these fatty acid synthase reactions are carried out by a single multifunctional protein, while in plant plastids and bacteria separate enzymes perform each step in the pathway. The fatty acids may be subsequently converted to triglycerides that are packaged in lipoproteins and secreted from the liver.
The synthesis of unsaturated fatty acids involves a desaturation reaction, whereby a double bond is introduced into the fatty acyl chain. For example, in humans, the desaturation of stearic acid by stearoyl-CoA desaturase-1 produces oleic acid. The doubly unsaturated fatty acid linoleic acid as well as the triply unsaturated α-linolenic acid cannot be synthesized in mammalian tissues, and are therefore essential fatty acids and must be obtained from the diet.
Triglyceride synthesis takes place in the endoplasmic reticulum by metabolic pathways in which acyl groups in fatty acyl-CoAs are transferred to the hydroxyl groups of glycerol-3-phosphate and diacylglycerol.
Terpenes and isoprenoids, including the carotenoids, are made by the assembly and modification of isoprene units donated from the reactive precursors isopentenyl pyrophosphate and dimethylallyl pyrophosphate. These precursors can be made in different ways. In animals and archaea, the mevalonate pathway produces these compounds from acetyl-CoA, while in plants and bacteria the non-mevalonate pathway uses pyruvate and glyceraldehyde 3-phosphate as substrates. One important reaction that uses these activated isoprene donors is steroid biosynthesis. Here, the isoprene units are joined together to make squalene and then folded up and formed into a set of rings to make lanosterol. Lanosterol can then be converted into other steroids such as cholesterol and ergosterol. |

|
|
|
|
Degradation
Degradation (W)
Beta oxidation is the metabolic process by which fatty acids are broken down in the mitochondria or in peroxisomes to generate acetyl-CoA. For the most part, fatty acids are oxidized by a mechanism that is similar to, but not identical with, a reversal of the process of fatty acid synthesis. That is, two-carbon fragments are removed sequentially from the carboxyl end of the acid after steps of dehydrogenation, hydration, and oxidation to form a beta-keto acid, which is split by thiolysis. The acetyl-CoA is then ultimately converted into ATP, CO2, and H2O using the citric acid cycle and the electron transport chain. Hence the citric acid cycle can start at acetyl-CoA when fat is being broken down for energy if there is little or no glucose available. The energy yield of the complete oxidation of the fatty acid palmitate is 106 ATP. Unsaturated and odd-chain fatty acids require additional enzymatic steps for degradation. |
|
|
|
| |
| |
Nutrition and health
Nutrition and health (W)
Most of the fat found in food is in the form of triglycerides, cholesterol, and phospholipids. Some dietary fat is necessary to facilitate absorption of fat-soluble vitamins (A, D, E, and K) and carotenoids. Humans and other mammals have a dietary requirement for certain essential fatty acids, such as linoleic acid (an omega-6 fatty acid) and alpha-linolenic acid (an omega-3 fatty acid) because they cannot be synthesized from simple precursors in the diet. Both of these fatty acids are 18-carbon polyunsaturated fatty acids differing in the number and position of the double bonds. Most vegetable oils are rich in linoleic acid (safflower, sunflower, and corn oils). Alpha-linolenic acid is found in the green leaves of plants, and in selected seeds, nuts, and legumes (in particular flax, rapeseed, walnut, and soy). Fish oils are particularly rich in the longer-chain omega-3 fatty acids eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA). Many studies have shown positive health benefits associated with consumption of omega-3 fatty acids on infant development, cancer, cardiovascular diseases, and various mental illnesses, such as depression, attention-deficit hyperactivity disorder, and dementia. In contrast, it is now well-established that consumption of trans fats, such as those present in partially hydrogenated vegetable oils, are a risk factor for cardiovascular disease. Fats that are good for you can be turned into trans fats by overcooking.
A few studies have suggested that total dietary fat intake is linked to an increased risk of obesity and diabetes. However, a number of very large studies, including the Women's Health Initiative Dietary Modification Trial, an eight-year study of 49,000 women, the Nurses' Health Study and the Health Professionals Follow-up Study, revealed no such links. None of these studies suggested any connection between percentage of calories from fat and risk of cancer, heart disease, or weight gain. The Nutrition Source, a website maintained by the Department of Nutrition at the Harvard School of Public Health, summarizes the current evidence on the impact of dietary fat: "Detailed research—much of it done at Harvard—shows that the total amount of fat in the diet isn't really linked with weight or disease." |

|
|
|
|

|
|
|
|
|
|

